The general hydro-carbon \(C_nH_m\) source for hydrogen results from the general steam-reformation reaction \(C_nH_m + n H_2O \rightarrow n CO + (n + m/2) H_2\) to deliver pure diatomic hydrogen \(H_2\).
 +
+  →
→
 + 3
+ 3 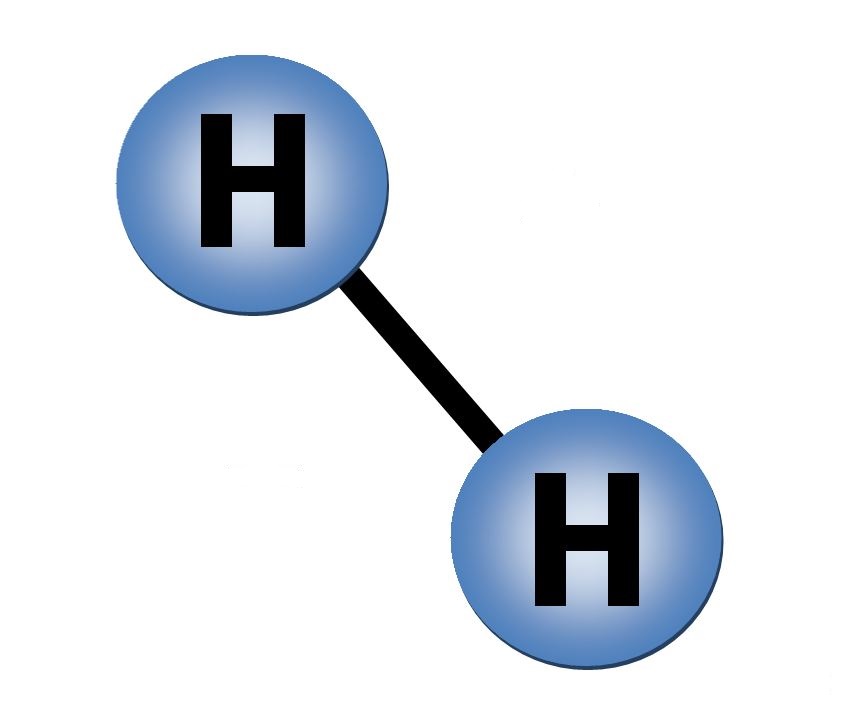
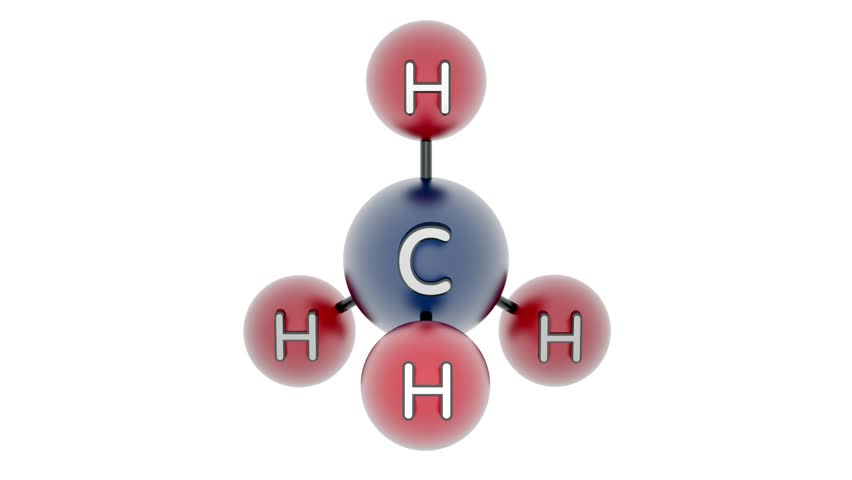
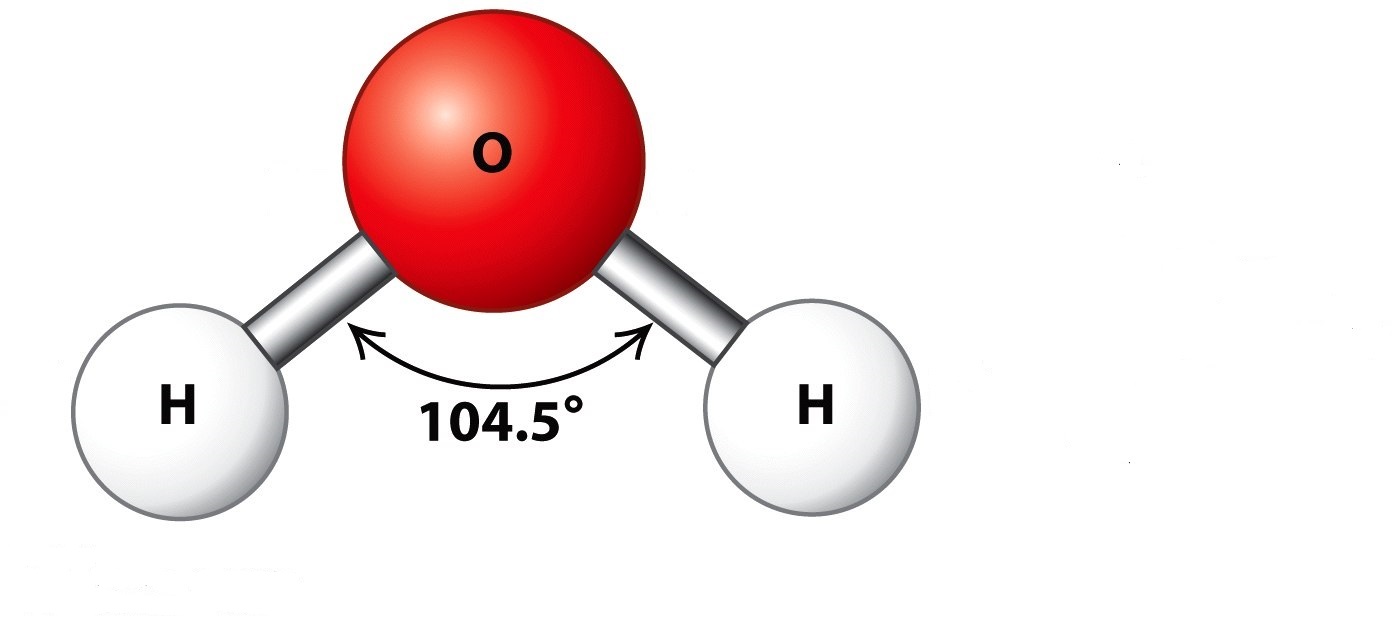
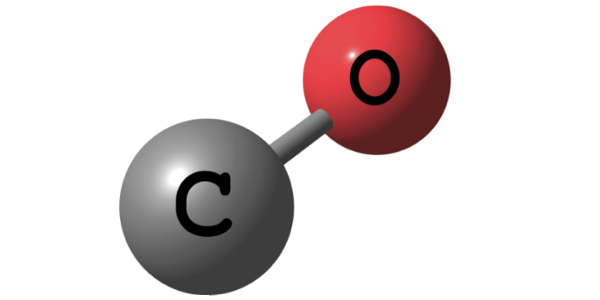
For the case of Wood alcohol or Methanol \(CH_3OH\) as hydrogen source material the steam-reformation reaction \(CH_3OH + H_2O \rightarrow CO + 3 H_2\) delivers the pure diatomic hydrogen \(H_2\).
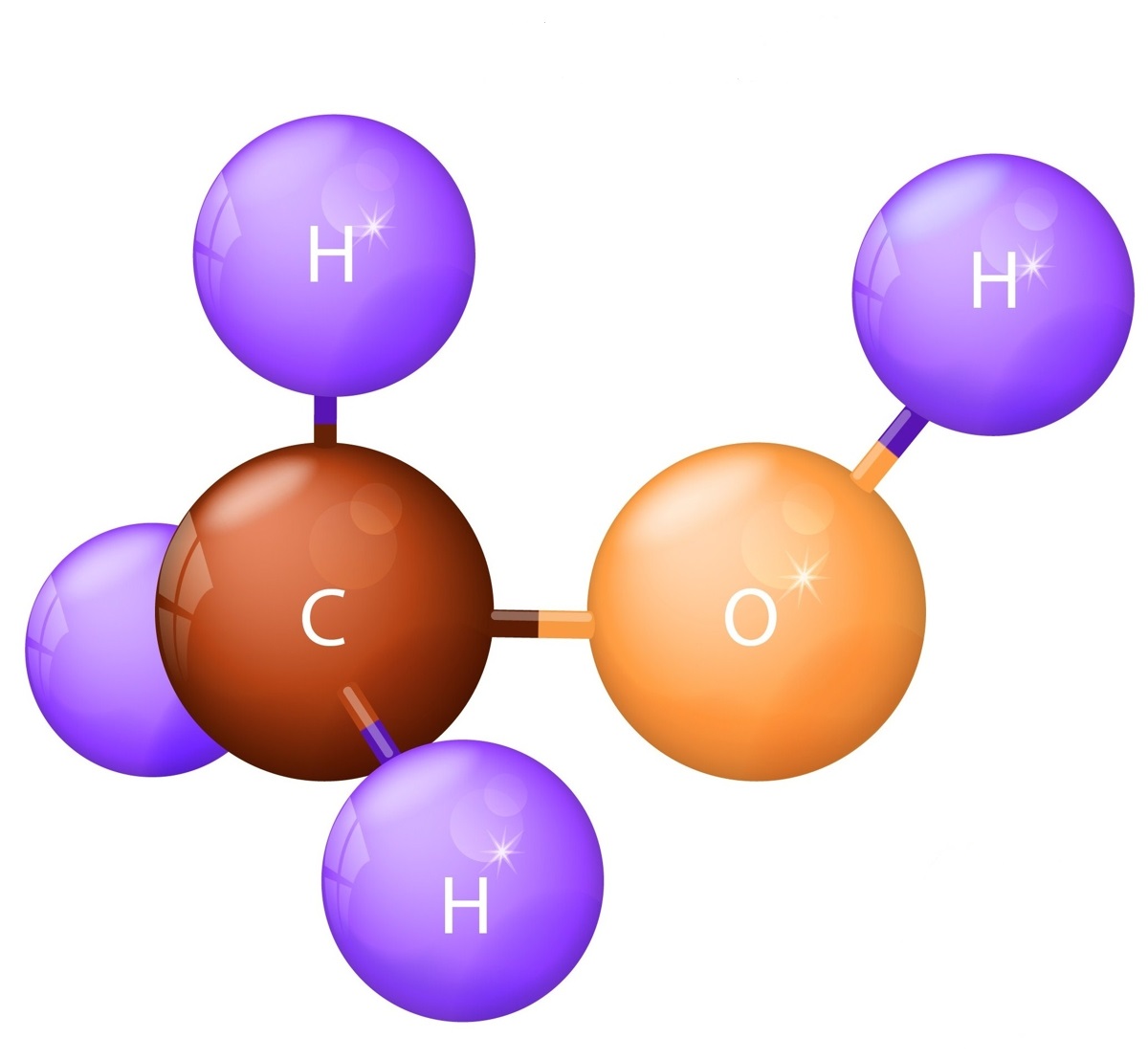 +
+  →
→
 + 3
+ 3 
For the case of Propane \(C_3H_8\) as hydrogen source material the steam-reformation reaction \(C_3H_8 + 3 H_2O \rightarrow 3 CO + 7 H_2\) delivers the pure diatomic hydrogen \(H_2\).
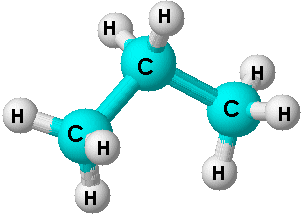 + 3
+ 3  →
3
→
3  + 7
+ 7 
The water-gas shift reaction \( CO + H_2O \rightarrow CO_2 + H_2 \) then further converts the \(CO\) into \(CO_2\) by water splitting to generate even more hydrogen.
 +
+  →
→
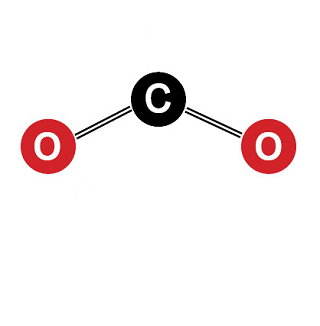 +
+ 